Introduction
The influence of pre-transplant leukemic load on relapse and long-term survival is well-documented among patients with B-cell acute lymphoblastic leukemia (B-ALL). Blinatumomab, a bispecific T cell engager, has shown promising results as an effective treatment for relapsed B-ALL. However, the dosage (28μg/day) and duration (28 days of continuous infusion) of blinatumomab have been predominantly determined in a relapsed leukemia context. Therefore, in the minimal residual disease (MRD) scenario, the standard dosing may exceed the required amount, potentially leading to increased toxicity, delays in transitioning to the scheduled transplant, and superfluous economic expenditure.
Methods
We enrolled B-ALL patients with ≤10% MRD who were slated for allogeneic hematopoietic stem cell transplantation (ASCT). MRD was quantified via multi-parameter flow cytometry or polymerase chain reaction (PCR) for the target gene. Blinatumomab therapy began at 8 μg/day, gradually escalating to 28 μg/day over only 5-10 days. Bone marrow cells were harvested post-blinatumomab therapy. Upon acquiring MRD negativity, patients proceeded to the transplantation protocol. All patients underwent myeloablative conditioning with chidamide, fludarabine, cytarabine, and busulfan. Peripheral stem-cell grafts were harvested from HLA-matched sibling donors (MSD), matched unrelated donors (MUD), or haploidentical donors (HID). Post-transplantation acute GVHD prophylaxis consisted of cyclophosphamide and cyclosporine. For HID transplant recipients, mycophenolate mofetil was added from day +5 to day +35. Follow-up examinations were scheduled at +1, +2, +3, +4, +6, +9, +12, +18, and +24 months post-transplant.
Results
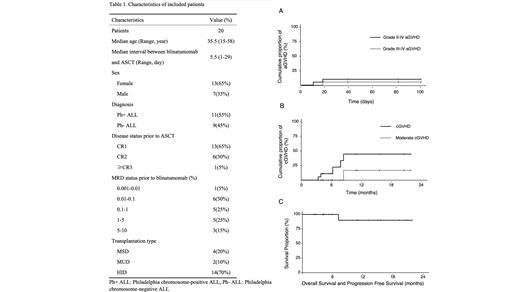
Our study cohort comprised 20 patients (13 females, 7 males), median age 35.5 years (range 15-58). Patient and disease characteristics are summarized in Table 1. During the blinatumomab therapy, 8 patients (40%) experienced grade 1 cytokine release syndrome (CRS), and 1 patient (5%) experienced grade 2 CRS, with no instances of grade 3 or higher CRS observed. All patients achieved complete remission (CR) and MRD clearance evaluated by flow cytometry following blinatumomab treatment. Eleven patients were diagnosed with Philadelphia chromosome-positive ALL, and 5 of them didn't achieve MRD clearance evaluated by PCR. Allo-SCT was conducted following blinatumomab at a median interval of 5.5 days (range 1-29) using MSD in 4 patients, MUD in 2 patients, and HID in 14 patients. The median time to neutrophil and platelet engraftment was 12 days (range 9-17) and 13 days (range 9-28), respectively. The median follow-up duration was 10.2 months (range 2-21.7). By day +30 post-ASCT, all patients achieved CR and MRD clearance with full donor chimerism. Acute GVHD (aGVHD) (grades II-IV) and (grades III-IV) incidences were recorded at 10.0% and 5.3% respectively (Figure 1A). One fatality occurred due to COVID-19-associated pulmonary aspergillosis 7.2 months post-transplantation. Among the 18 evaluable patients, the estimated 1-year cumulative incidence of chronic GVHD (cGVHD) was 44.4%, with no instances of severe cGVHD observed (Figure 1B). No patient relapsed at the last follow-up on July 31, 2023. The estimated 1-year progression-free survival (PFS) and overall survival (OS) both stood at 90% (Figure 1C).
Conclusion
Short-term blinatumomab as a bridging therapy for adult patients with low-burden B-ALL transitioning to ASCT demonstrates comparable efficacy to standard dosing regarding disease control and survival outcomes.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal